Please allow ads on our site
Please log in to access this content. You will be redirected to the login page shortly.
LoginArrange the following compounds in the decreasing of their boiling points and solubilitiy in H_(2)O.
a.(I) Methanol(II) Ethanol(III) Propan-1-ol(IV) Butan-1-oI(V) Butan-2-oI(VI) Pentane-1-oI
b. (I) Pentanol(II) n-Butane(III) Pentanal(IV) Ethoxy ethane
c. (I) Pentane(II) Pentane-1,2,3,-triol(III) Butanol
d. (I) Butane(II)Butanol(III) Pentanol
e. (I) Pentan-1-oI(II)2-Methyl butan-2-oI(III)3-Methyl butan-2-oI
f.(I) n-Butyl alcohol(II) sec-Butyl alcohol(III)t-Butyl alcohol
Class 12 Chemistry in Class 12 3 years ago
Solubility order:`I gt II gt III gt V gt IV gt VI`
Explanation:All of them are alcohols, so all have H-bonding. As the molecular mass and surface area increase, the boiling point increase and solubility decreases.Out of (IV) and (V) , there is branching in (V) and has less surface area than (IV), so the boiling of (IV) gt (V), but solubility of (V) gt (IV).
b.Boiling point order: `I gt III gt IV gt II
Solubility order:`I gt III gt IV gt II`
In (I), there is H-bonding, in (II) (aldehyde), dipoledipole INTERACTION, in (III) (ether), slightly polar due to EN of O, and in (IV) (alkane), van der Waals interaction (non-polar).
c.Boiling point order:`II gt III gt I`
Solubility order:`II gt III gt I`
In (II), three `(---OH)` GROUND, more H-bonding, in (II), one Waals interaction.
d.Boiling point order:`III gt II gt I`
Solubility order:`II gt III gt I`
Both (II) and (III) have H-bonding, but molecular mass of (III) gt (II), hence the given boiling point order. Solubility of (II) gt (III), because in (III), size of R-group non-polar (hydrophobic part) is larger, hence the given solubility order.
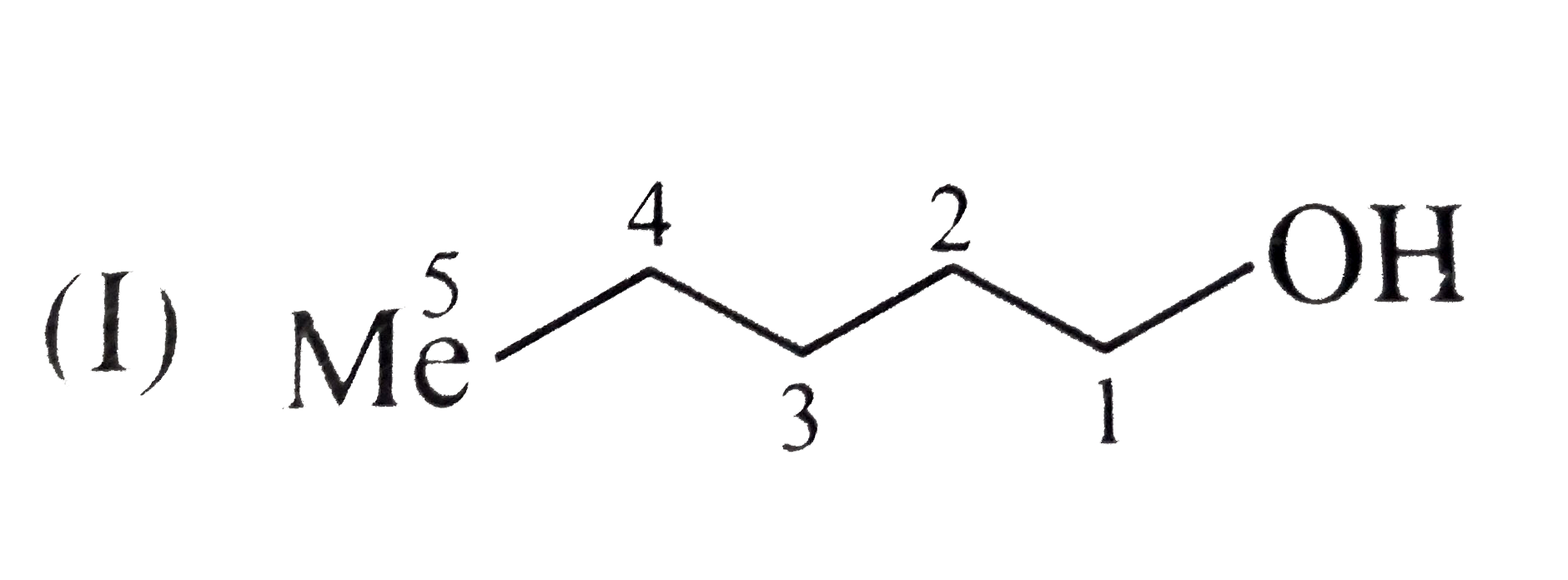
e.Boiling point order:`I gt II gt III`
Solubility order :`III gt II gt I`
(I)
 (II)
(II)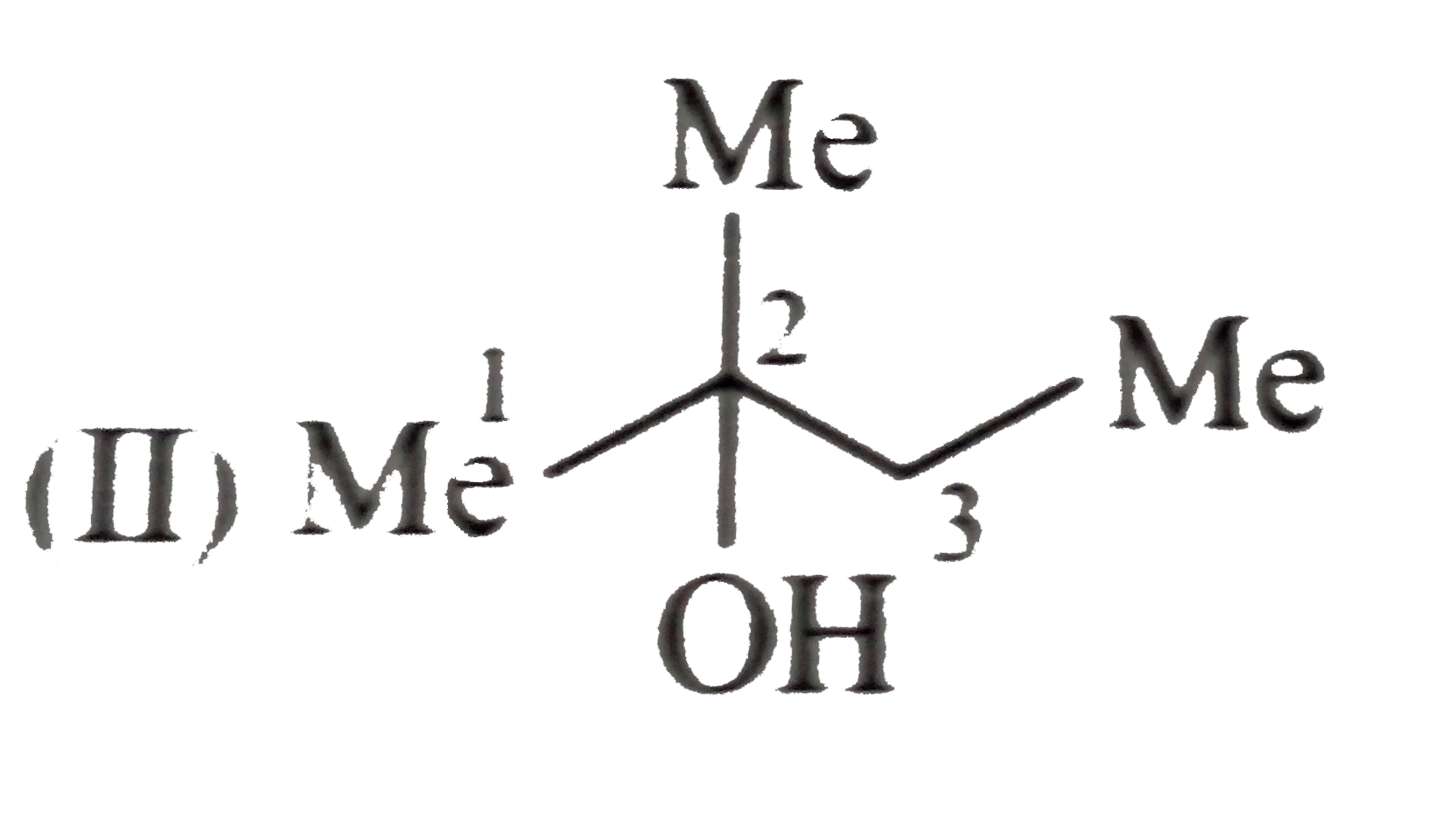
(III)
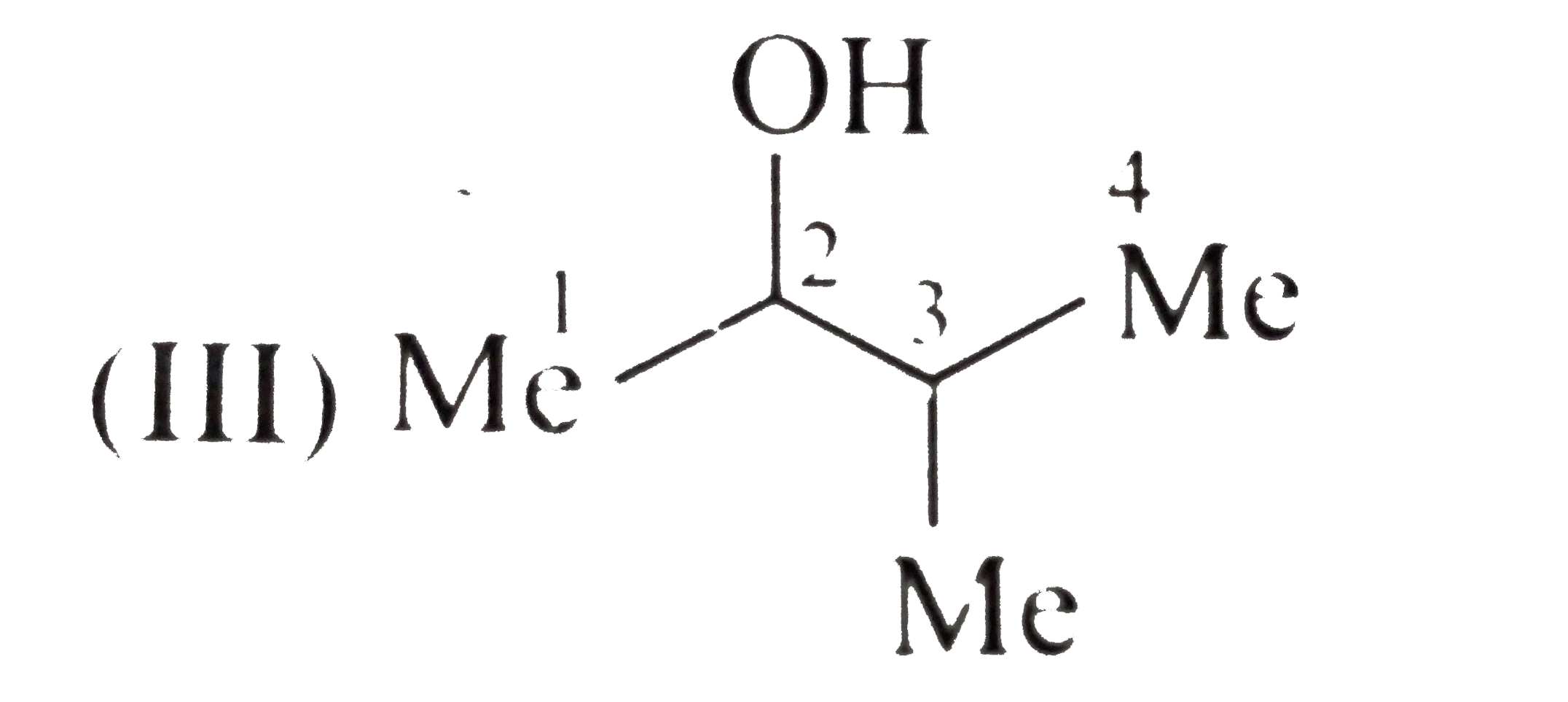
All alcohols have H-bonding, same molecular MASSES, but branching increases form(I) to (II). Their shape becomes more compact and spherical and therefore less surface contact is available for van der Waals attractive FORCE. So, boiling point decreases and solubility in `H_(2)O` increase.
f.Boiling point order :`I gt II gt III`
Solubility order :`III gt II gt I`
All aclcohols have H-bonding. Surface area of `(I) gt (II) gt III` . Hence, the boiling point and solubility order are as given above.
Posted on 04 Dec 2021, this text provides information on Class 12 related to Chemistry in Class 12. Please note that while accuracy is prioritized, the data presented might not be entirely correct or up-to-date. This information is offered for general knowledge and informational purposes only, and should not be considered as a substitute for professional advice.
Take Quiz To Earn Credits!
Turn Your Knowledge into Earnings.

No matter what stage you're at in your education or career, TuteeHub will help you reach the next level that you're aiming for. Simply,Choose a subject/topic and get started in self-paced practice sessions to improve your knowledge and scores.

